Participant Resources

IRB
- This study has been approved by the Ohio State University Institutional Review Board, Protocol #2024H0193
- The formal name of the study is “Comparative Effectiveness Trial of Metformin versus Insulin for the Treatment of Gestational Diabetes”
- The Primary Investigators for the study are Dr. Mark Landon and Dr. Kartik Venkatesh

Informed Consent
As a participant, you should have received a copy of the Study Informed Consent Form which includes important information about the study and what to expect. If you have lost or misplaced this form, you may request a new copy by contacting the appropriate study team here.

Incentives
Participants will receive these incentives:
- $200 at the start
- $50 at 6 weeks after birth
- $125 at 2 years after birth
- An extra $50 for those who are chosen for and do an interview at 6 weeks after birth
If you are expecting but have not received your participation incentives, please contact the appropriate study team here.
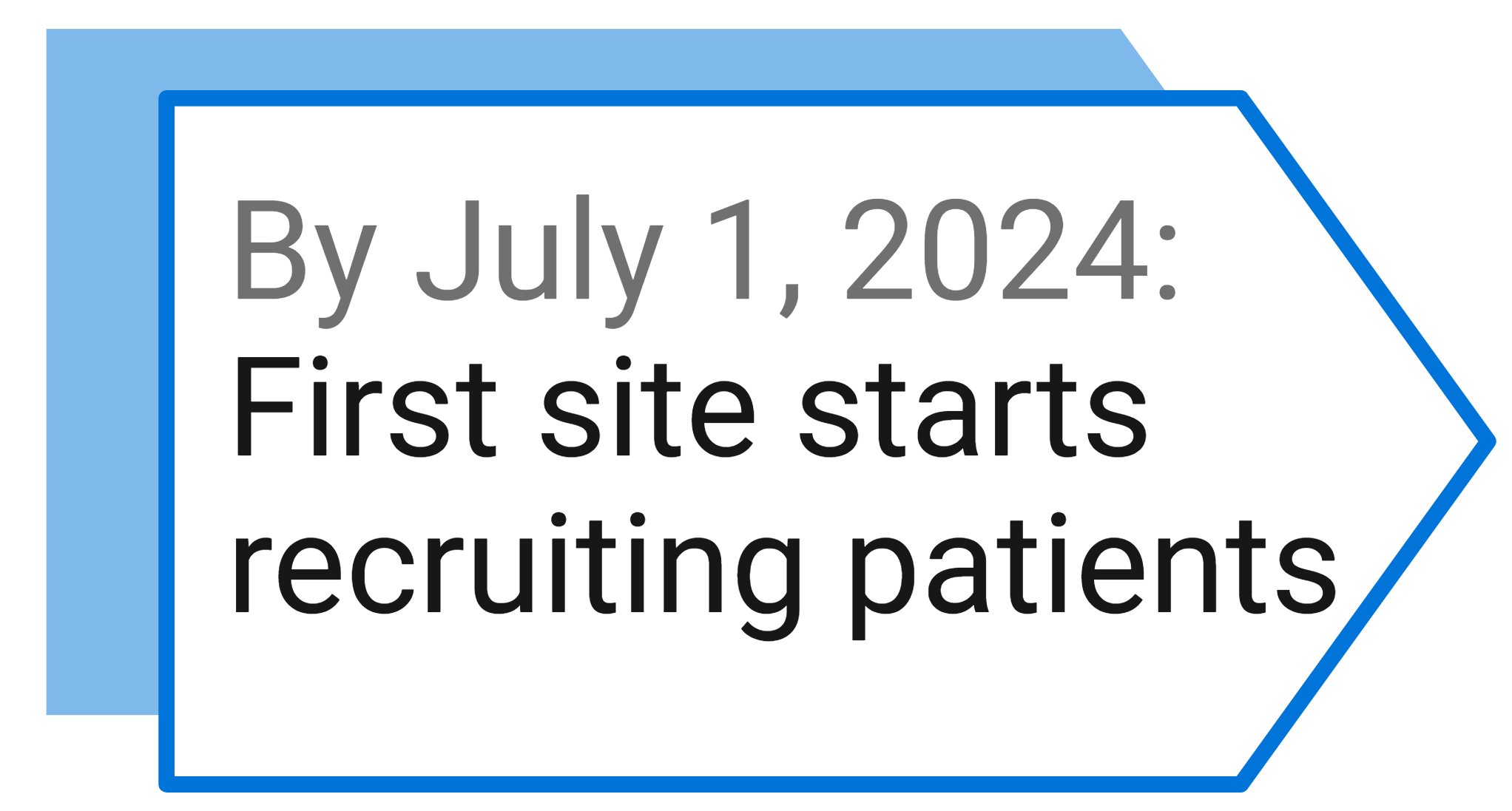
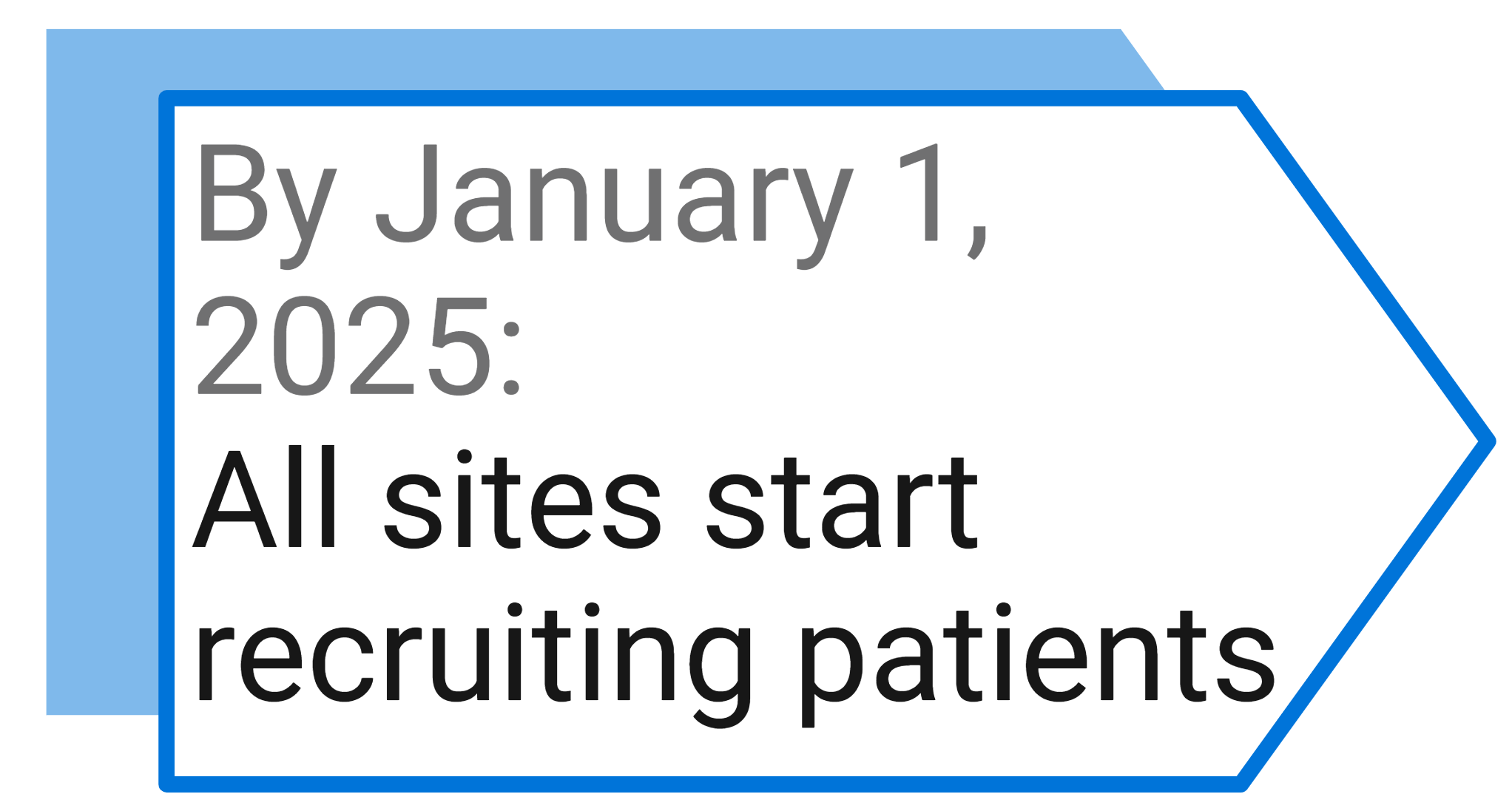
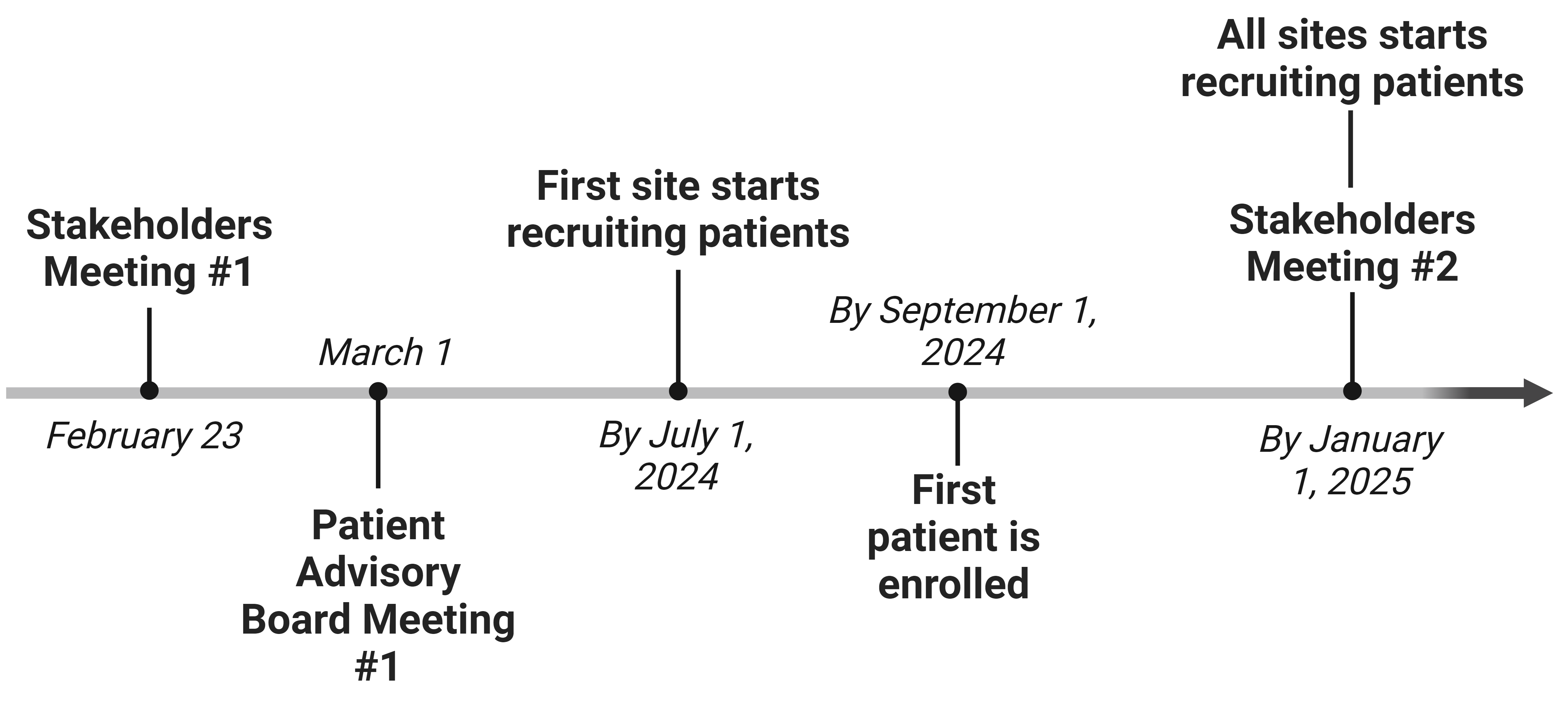
Study Milestones and Events